In the diagnosis and treatment of diabetes, we are accustomed to the dichotomy of “either-or” : either type 1 diabetes (T1DM) or type 2 diabetes (T2DM). However, in clinical practice, there are always some cases that puzzle doctors: some adult patients who seem to have “type 2 diabetes” at the onset quickly progress to insulin dependence; Some patients have been treated for type 2 diabetes for many years, but they develop a tendency towards ketosis after a certain infection or stress. Behind these cases lies a type of Diabetes that has long been underestimated – Latent Autoimmune Diabetes in Adults (LADA).
1. LADA: The Neglected “Middle Ground” blood glucose analyzer
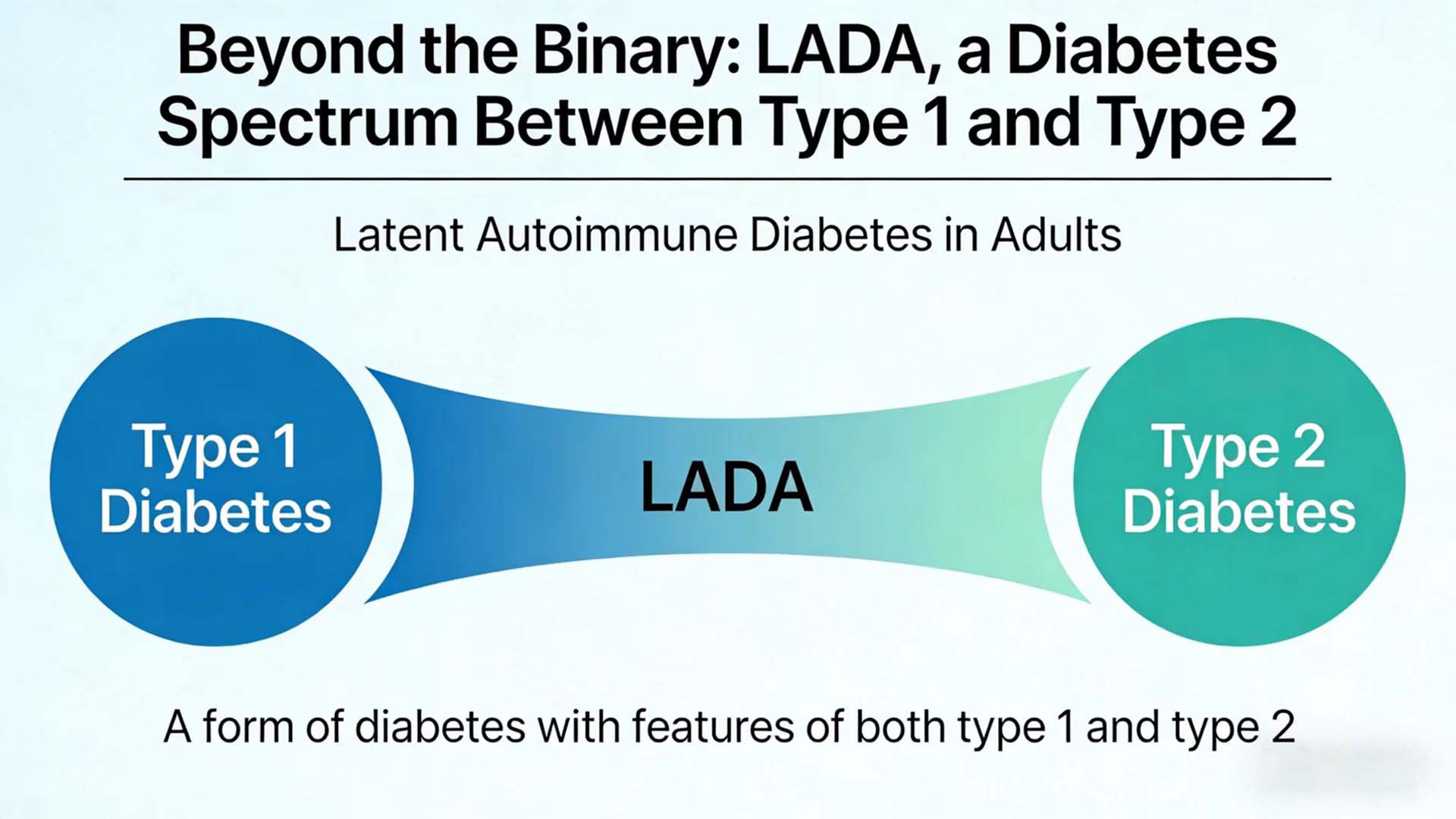
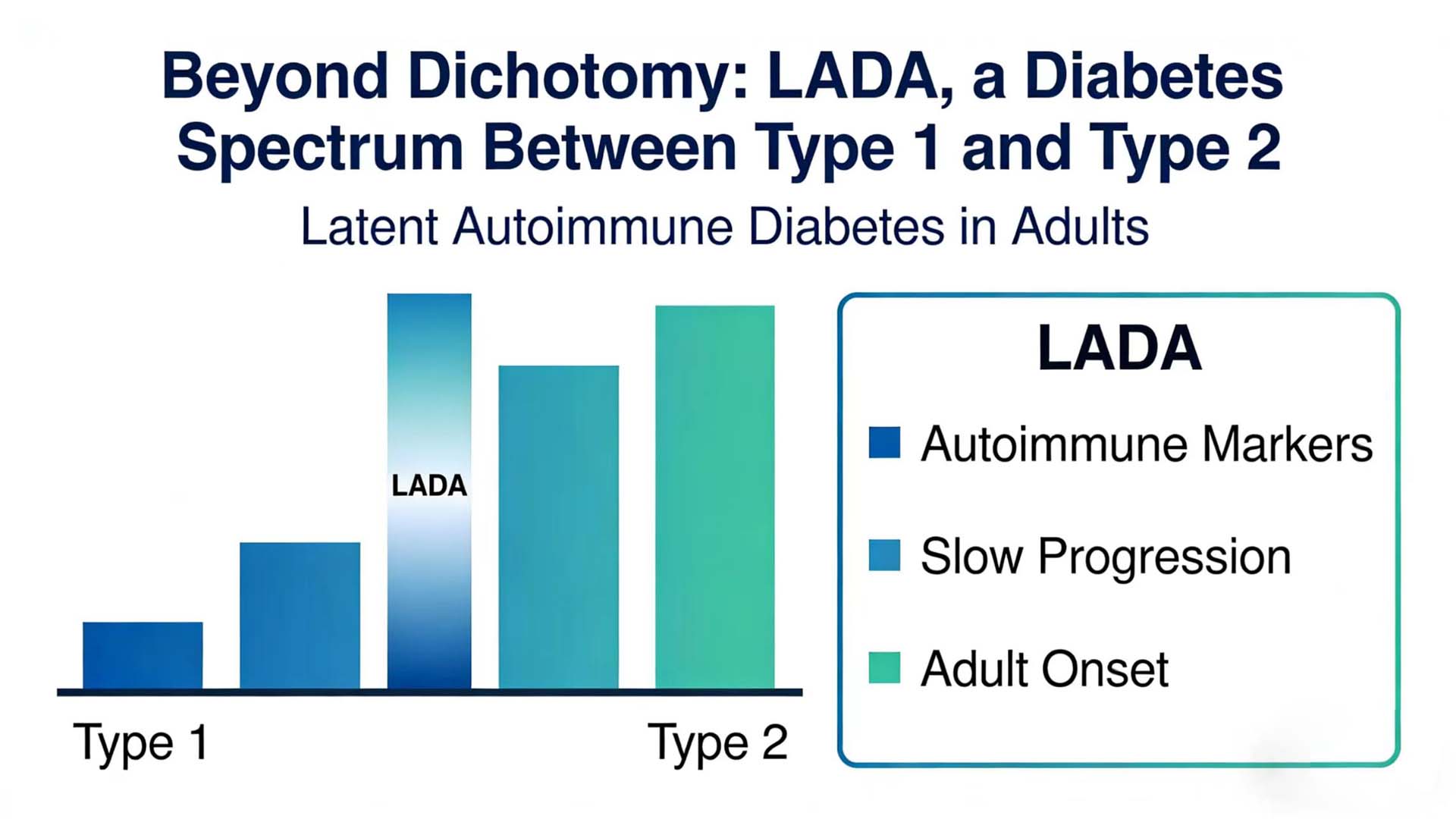
LADA is a special type of diabetes that straddles between type 1 and type 2 diabetes, combining the characteristics of both but not entirely belonging to either type. In recent years, with the deepening understanding of the autoimmune mechanism and the advancement of detection technology, the diagnosis and treatment model of LADA is undergoing a profound transformation from “empirical diagnosis” to “precise classification”.
1.1 Definition and Classification: The Cognitive Evolution from “Hidden” to “Dynamic”
LADA refers to a type of diabetes in which the age of onset is ≥18 years (or 30 years, with slight differences in different guideline standards), there is no obvious tendency towards ketosis at the onset, but there is evidence of autoimmune damage to pancreatic β cells. Its core feature lies in its “insidious” nature – the clinical manifestations at the onset are similar to those of type 2 diabetes, but the autoimmune process continues to progress, and eventually most patients will rely on insulin treatment.
The International Diabetes Federation (IDF) and the American Diabetes Association (ADA) have not yet classified LADA as an independent classification. However, in clinical practice, based on whether autoantibodies are positive or not, adult diabetes can be simplified into “autoimmune-positive” (including type 1A diabetes and LADA) and “autoimmune-negative” (mainly T2DM). The key differences between LADA and type 1A diabetes lie in the age of onset, the mode of onset and the rate of β -cell function decline: the former has a later onset and a slower progression, while the latter is more common in children and adolescents, with an acute onset and rapid β -cell function failure.
In recent years, scholars have further proposed the concept of “subtyping” for LADA: based on the age of onset (such as ≤35 years old vs.>35 years old) and the number of positive antibodies (such as single antibody positive vs. LADA is classified into “slowly progressive type” and “rapidly progressive type” based on factors such as multiple antibody positivity and the degree of β -cell functional residual (such as C-peptide level). This classification has significant guiding significance for the selection of treatment strategies – for instance, patients with multiple positive antibodies and low C-peptide levels may initiate insulin treatment earlier.
1.2 Epidemiological Data: The Underestimated “Tip of the Iceberg”
The prevalence of LADA in adult diabetes varies due to different diagnostic criteria, especially the combination of age cutoff value and antibody testing, but generally ranges from 10% to 25%. The LADA China Study shows that among newly diagnosed diabetic patients over 18 years old, the positive rate of GADAb is 5.9%-12.6%. If combined with IA-2Ab detection, the positive rate can increase to 8%-10%.
It is worth noting that the prevalence of LADA decreases with the increase of the age of onset: among newly diagnosed diabetes patients aged 18 to 30, LADA accounts for approximately 20%. Among people over 60 years old, it still accounts for 5% to 10%. This reminds us that even among middle-aged and elderly diabetic patients, autoimmune factors should not be ignored. In addition, LADA patients often have metabolic abnormalities (such as obesity and dyslipidemia), but the degree of obesity is usually lower than that of T2DM patients. Moreover, indicators such as waist circumference and BMI are negatively correlated with antibody titers – that is, the thinner the body shape and the milder the metabolic disorder, the more prominent the autoimmune damage may be.
1.3 Clinical Significance: Why is Early identification of LADA crucial? blood glucose kit
Misdiagnosis or missed diagnosis of LADA will lead to deviations in treatment strategies and affect the prognosis of patients. On the one hand, if LADA is misdiagnosed as T2DM, long-term use of insulin sensitizers (such as metformin) or secretants (such as sulfonylureas) may not effectively delay the decline of β -cell function and may even accelerate its failure. On the other hand, the risk of cardiovascular complications, microvascular lesions (such as diabetic nephropathy and retinopathy), and neuropathy in LADA patients is significantly higher than that in T2DM patients, which may be jointly related to chronic hyperglycemia, autoimmune inflammation, and insufficient insulin secretion.
The significance of early identification of LADA lies not only in correcting treatment plans but also in providing “individualized early warnings” for patients. For instance, after a clear diagnosis of LADA, it is necessary to enhance blood glucose monitoring, avoid factors that may trigger ketosis (such as infection and stress), and conduct early screening for diabetic complications. In addition, LADA, as an “intermediate type” of autoimmune diabetes, provides a natural model for studying the pathogenesis of type 1A diabetes and is conducive to exploring the “time window” of immune intervention.
2. The pathogenesis of LADA: A “triangular game” among Genetics, immunity and environment
The pathogenesis of LADA is complex, involving the interaction of multiple factors such as genetics, immunity and environment, forming a complex “triangular game”.
2.1 Genetic Background: The “Dual Role” of Susceptibility Genes
The genetic susceptibility of LADA combines the characteristics of type 1A diabetes and type 2 diabetes mellitus. Human leukocyte antigen (HLA) genes are the most important genetic factors among them, especially the polymorphism of HLA-DR/DQ loci. Similar to type 1A diabetes, the frequencies of HLA-DR3, DR4 and their heterozygotes (DR3/DR4) are significantly increased in patients with LADA. However, unlike type 1A, the frequency of HLA-DR2 is decreased in LADA patients, while the frequency of HLA-DR9 (common in the Asian population) is increased – this may be the genetic basis for the high incidence of LADA in the Asian population.
Non-hla genes are also involved in the pathogenesis of LADA. The lymphophosphatase encoded by the PTPN22 gene can affect the autoimmune response by regulating T cell activation, and its rs2476601 polymorphism (R620W) is associated with the risk of LADA. The rs231775 polymorphism of the CTLA-4 gene (a key gene for immune regulation) is also associated with the susceptibility to LADA. In addition, T2DM susceptibility genes (such as TCF7L2 and KCNJ11) also have mutations in LADA patients, suggesting that the “superimposed effect” of genetic background may determine the clinical phenotype of LADA.
2.2 Autoimmunity: A cascade reaction from “initiation” to “attack”
The core pathophysiological feature of LADA is autoimmune damage to pancreatic β cells, a process similar to that of type 1A diabetes, but with a slower progression. It is currently believed that the autoimmune response of LADA begins with environmental factors triggering it (such as viral infection, dietary factors), which activate antigen-presenting cells (such as dendritic cells) in genetically susceptible individuals. Subsequently, the activated CD4+T cells assist B cells in producing autoantibodies (such as GADAb, IA-2Ab), while CD8+T cells directly kill β cells.
Autoantibodies are the “markers” of immune damage in LADA and also the core basis for clinical diagnosis. Among them, glutamic acid decarboxylase antibody (GADAb) has the highest sensitivity (over 90%) and the longest duration. The positive rates of islet antigen 2 antibody (IA-2Ab), insulin autoantibody (IAA, more common in young patients), and zinc transporter 8 antibody (ZnT8Ab) decreased successively. Combined detection of multiple antibodies can increase the detection rate of LADA – for instance, those who are positive for both GADAb and IA-2Ab antibodies have a faster rate of β -cell function decline, and their insulin dependence rate can reach 80% within five years.
2.3 Environmental Factors: The “Last Straw” That Triggers Immunity
The role of environmental factors in the pathogenesis of LADA is not yet fully understood, but it is currently believed that the “hygiene hypothesis” and the “molecular simulation hypothesis” may be involved. The “Hygiene Hypothesis” holds that a reduction in infections during childhood (such as excessive cleaning and antibiotic abuse) may lead to an overactivated immune system that is prone to attacking its own tissues. The “molecular simulation hypothesis” suggests that the antigenic components of certain viruses (such as Coxsackievirus and mumps virus) are similar to β -cell antigens, and the T cells activated after infection may “accidentally damage “β -cells.
In addition, dysbiosis of the intestinal flora may be involved in the pathogenesis of LADA through the “gut-pancreatic axis”. Research has found that the diversity of the intestinal microbiota in LADA patients is reduced, with a decrease in short-chain fatty acid-producing bacteria (such as Clostridium pratidis) and an increase in pathogenic bacteria (such as Enterobacteriaceae). Metabolic products of the microbiota (such as lipopolysaccharides) may activate local inflammatory responses in the islets through Toll-like receptors and accelerate β -cell damage. blood glucose measurement
3. Diagnosis of LADA: From “Empirical Judgment” to “Combined Validation of Immune Markers”
Early diagnosis is the key to LADA management. With the advancement of detection technology, the diagnosis of LADA has shifted from “empirical judgment” to “combined verification of immune markers”.
3.1 Diagnostic Criteria: From “Single Indicator” to “Comprehensive Judgment”
According to the “Chinese Expert Consensus on the Diagnosis and Treatment of Latent Autoimmune Diabetes in Adults (2021 Edition)”, the diagnostic criteria for LADA are:
The age of onset is ≥18 years old.
Positive for islet autoantibodies or positive for islet autoimmune T cells;
No insulin treatment should be relied on for at least half a year after the diagnosis of diabetes.
Compared with the old version of the consensus, the new version of the consensus has included positive pancreatic autoimmune T cells in the diagnostic criteria, which is helpful for identifying patients with negative antibodies but autoimmune damage. In addition, the new consensus suggests screening for glutamic acid decarboxylase autoantibodies (GADA) for all newly diagnosed T2DM patients. For patients with negative GADA antibodies, if they are clinically suspected of having LADA, further screening for other autoantibodies or islet antigen-specific T cells should be conducted.
3.2 Diagnostic Pathway: From “Passive Discovery” to “Active Screening”
The diagnostic pathway for LADA recommended in the new consensus is:
Routine screening for GADA was conducted for all newly diagnosed T2DM patients.
For patients with positive GADA, LADA is diagnosed in combination with the age of onset and insulin dependence.
For patients with GADA negative but clinically suspected LADA (such as those with a family history of type 1 diabetes, BMI For patients diagnosed with LADA, further assessment of pancreatic β -cell function, autoantibody titers and comorbidities should be conducted to guide treatment decisions. blood glucose measurement
3.3 Differential Diagnosis: Avoid the misconception of “either-or”
The differential diagnosis of LADA mainly includes:
Type 1A diabetes: The age of onset is usually less than 30 years old, with an acute onset. It is often accompanied by ketoacidosis, rapid failure of pancreatic β -cell function, and a higher positive rate of autoantibodies.
Type 2 diabetes: It has an insidious onset, often presenting with metabolic syndrome manifestations such as obesity and insulin resistance. Autoantibodies are negative, and the decline of β -cell function is slow.
Other special types of diabetes, such as mitochondrial diabetes and MODY, can be identified through genetic testing and other means.
4. Treatment of LADA: Strategy Upgrade from “controlling blood sugar” to “protecting beta cells”
The therapeutic goal of LADA is not only to control blood sugar, but more importantly, to regulate the autoimmune response of the islets, protect the function of pancreatic β cells, and prevent complications and comorbidities of diabetes.
4.1 Treatment Principles: Individualized and stratified management
According to the “Chinese Expert Consensus on the Diagnosis and Treatment of Latent Autoimmune Diabetes in Adults (2021 Edition)”, the treatment of LADA should be individualized and stratified based on C-peptide levels, GADA titers and comorbidities.
If C-peptide is less than 0.3 nmol/L or the GADA titer is ≥180 U/ml: insulin treatment is recommended.
If C-peptide is ≥0.3 nmol/L and GADA titer is
4.2 Drug Selection: Avoid harmful substances and prefer beneficial ones
The selection of drugs for LADA should follow the following principles:
Avoid using sulfonylurea drugs: Sulfonylurea drugs can stimulate pancreatic beta cells to secrete insulin, which may accelerate the failure of beta cell function.
Insulin therapy: For patients with poor pancreatic islet function and high GADA titer, insulin therapy should be used early to protect the residual β -cell function.
Oral hypoglycemic drugs: For patients with good pancreatic islet function and low GADA titer, metformin, DPP-4 inhibitors, GLP-1 receptor agonists, SGLT2 inhibitors and other drugs can be selected, but sulfonylurea drugs should be avoided.
Immunomodulatory therapy: Currently, immunomodulatory therapy is still in the research stage, such as vitamin D and immunosuppressants, which may have a certain protective effect on pancreatic islet function, but further verification is needed.
4.3 Lifestyle Intervention: The foundation of foundations
Lifestyle intervention is the foundation of LADA treatment, including dietary control, moderate exercise, weight management, smoking cessation and alcohol restriction, etc. Studies show that a healthy lifestyle can improve insulin resistance, reduce the burden on pancreatic beta cells and delay the progression of the disease.
5. Future Outlook: Moving towards the “Precision Medicine” era of LADA
With the continuous advancement of medical technology, the diagnosis and treatment of LADA will face more opportunities and challenges
Precise typing: By leveraging techniques such as genetic testing, metabolomics, and proteomics, the typing of LADA is further refined to provide a basis for individualized treatment.
Early screening: Develop more sensitive and specific autoantibody detection methods, or utilize artificial intelligence technology to analyze clinical data to achieve early screening and diagnosis of LADA.
Immunotherapy: Explore specific immunotherapy methods for LADA, such as antigen-specific immune tolerance and immune cell therapy, to prevent or reverse autoimmune damage.
β -cell regeneration: Research on β -cell regeneration technologies, such as stem cell transplantation and gene editing, to provide new treatment options for LADA patients.
LADA is a special type of diabetes that straddles the line between type 1 and type 2 diabetes, challenging our dichotomous understanding of diabetes as “either-or”. As our understanding of LADA deepens, we gradually realize that diabetes is not simply “type 1″ or “type 2″, but a continuous disease spectrum, and LADA is an important part of this spectrum.
Early identification, precise classification and individualized treatment are the keys to LADA management. As clinicians, we need to break the “either-or” mindset, pay attention to those “atypical” cases of diabetes, identify LADA patients at an early stage through means such as immune marker detection, and formulate individualized treatment plans based on their clinical characteristics and disease progression.
In the future, with the continuous development of precision medical technology, we are expected to achieve early screening, precise diagnosis and effective treatment of LADA, bringing better health outcomes to this special group of people. Let’s go beyond the dichotomy and re-understand the disease spectrum of diabetes to provide more precise and effective medical services for LADA patients.
Post time: Feb-09-2026